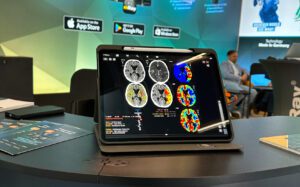
Wir freuen uns, bekannt zu geben, dass mRay nun offiziell in Brasilien als Medizinprodukt zertifiziert wurde! Die Zulassung durch ANVISA, die brasilianische Gesundheitsbehörde, stellt einen wichtigen Meilenstein für uns dar und eröffnet neue Möglichkeiten auf dem südamerikanischen Markt.
Warum ist die ANVISA-Zulassung so wichtig?
ANVISA (Agência Nacional de Vigilância Sanitária) ist die zentrale Behörde für die Regulierung und Überwachung von Medizinprodukten, Arzneimitteln und Gesundheitsdienstleistungen in Brasilien. Ohne eine ANVISA-Zertifizierung ist der Vertrieb von Medizinprodukten im brasilianischen Markt nicht möglich. Die Genehmigung bestätigt, dass mRay alle regulatorischen Anforderungen erfüllt und in Brasilien sicher eingesetzt werden kann.
Erstmalige Teilnahme an der Hospitalar in São Paulo
Mit der erfolgreichen Zertifizierung steht unserer Teilnahme an der Hospitalar 2025 nichts mehr im Wege! Die Hospitalar ist eine der bedeutendsten Gesundheitsmessen Lateinamerikas und bringt Fachleute aus der ganzen Welt zusammen. Vom 21. bis 24. Mai 2025 präsentieren wir mRay in São Paulo und freuen uns darauf, mit Expert*innen der Branche in den Austausch zu gehen.
Wir freuen uns auf spannende neue Möglichkeiten in Brasilien und darauf, mRay bald auch in südamerikanischen Gesundheitseinrichtungen verfügbar zu machen!
—
Tagungsort
São Paulo Expo
Rodovia dos Imigrantes
Vila Água Funda
São Paulo – SP, 04329-900
Brasilien
—
Termin
20. – 23. Mai 2025