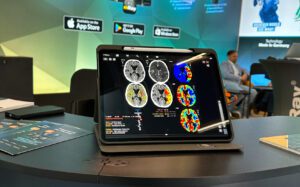
Als einer der ersten Medizinprodukte-Hersteller
Ursrpünglich war der 26. Mai 2020 Starttermin des Geltungsbereichs der neuen Medical Device Regulation (MDR) EU 2017/745. Auf Grund der Corona-Krise wurde die neue Verordnung über Medizinprodukte um ein ganzes Jahr verschoben, da eine Umstellung auch unter Regelbetrieb viele Medizintechnik-Unternehmen vor große Herausforderungen gestellt hätte, die man ihnen während der Ausnahmesituation durch Covid-19 nicht noch zusätzlich aufbürden wollte.
mbits hatte bereits vor der Corona-Krise das Qualitätsmanagement auf MDR umgestellt. Die just erschienene neue Version unserer App mRay 6.0 ist bereits ausnahmslos nach MDR-Richtlinien entwickelt worden.
Die Umstellung bedarf einiger grundlegender Anpassungen bestehender Workflows – eine große Herausfoderung. Das Soforthilfe-Programm der Gesundheitsindustrie BW erarbeitet gemeinsam mit Unternehmen gruppenspezifische Inhalte und stellt diese zur Verfügung.